MHRA research: better consultations, better evidence
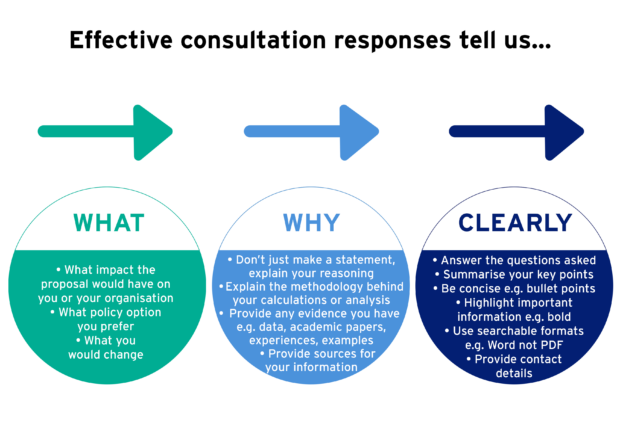
MHRA regularly consults its stakeholders on many issues. As Head of Economics I use the consultation responses to gather new evidence to see if we’ve got our policy right, or if we need to change it. It is extremely satisfying …